Health
Philips Launches New Handheld Blood Test for Rapid Point-of-Care Diagnosis of Heart Attack

- Launch in selected countries in Europe follows CE marking for Philips Minicare I-20 system for cardiac troponin testing
- Handheld Minicare I-20 system for use in emergency departments provides results in 10 minutes from a droplet of blood instead of the current one-hour wait and supports ruling out a possible heart attack in three hours
Royal Philips (NYSE: PHG, AEX: PHIA) announced the launch of a new handheld blood test, the Minicare I-20 system, for rapid diagnosis of a heart attack at the point of care. The new test is being introduced in selected countries in Europe including the UK, Germany, the Netherlands and Belgium, with introduction in other European countries following in due course.* As a result, patients with chest pain presenting at the emergency department are set to benefit from this major innovation, which Philips has recently CE marked for compliance with the European in vitro diagnostic medical devices directive. The handheld Minicare I-20 system measures the level of cardiac troponin I (cTnI), a protein that is excreted by the heart muscle into the blood following a heart attack. It delivers test results, comparable with those obtained by laboratory testing, in less than 10 minutes near the patient, reducing the time for a physician to decide on the appropriate treatment pathway.
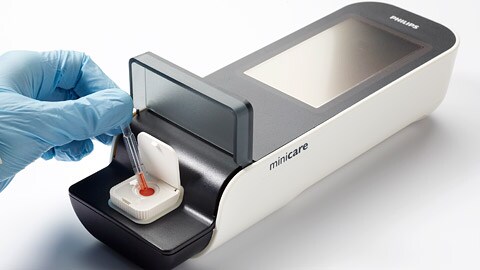
The Minicare I-20 system is the latest innovation to come out of Philips’ global R&D and new business development programs in the area of point-of-care testing and monitoring applications for the hospital and the home. It consists of a connected handheld analyzer, dedicated software, and a single-use disposable cartridge containing an application specific test based on Philips’ proprietary biosensor technology.
“The commercial launch of the Minicare I-20 system for cardiac troponin I testing represents a major milestone for us,” said Marcel van Kasteel, CEO of Handheld Diagnostics at Philips. “I am convinced that we will be able to make a real difference for patients and care providers. Minicare I-20 is designed to help care providers reduce both the time-to-treatment and time-to-discharge of patients, thereby helping to reduce crowding in emergency departments and improve the utilization of hospital resources.”
“Blood samples are usually analyzed in the hospital laboratory, and it can easily take more than an hour to get the result back to the emergency department physician,” said Dr. Paul Collinson, Consultant Chemical Pathologist at St George’s University Hospitals NHS Foundation Trust (UK). “Point-of-care testing can significantly help to reduce the turnaround time.”
The Minicare I-20 was tested in real-life acute care settings within the European Lab2Go project, a consortium of European hospitals. The study showed the potential of Philips Minicare cTnI system to accurately measure cTnI values, near the patient in the emergency department, with a turnaround time of less than 10 minutes.
* The Philips Minicare I-20 analyzer and Minicare cTnI test cartridges are sold in selected countries in Europe, including Austria, Belgium, Denmark, Germany, the Netherlands, Norway, Sweden, UK and Switzerland. Other European countries will follow in due course. The Philips Minicare I-20 analyzer and Minicare cTnI test cartridges are not available for sale in the US.